Le basi per una corretta crioconservazione di campioni biologici
Methods for the Effective Preservation of Cell and Tissue Samples
The use of preservation techniques, both cryopreservation and hypothermic storage, is widespread both in laboratory and clinical settings. While commonplace, most often the specific protocols and process utilized for preserving cells are based on dated information and often are lacking in detail. As such, many of the protocols applied today provide suboptimal results and often do not incorporate modern day preservation media, which can offer significant improvements over serum/DMSO cocktails. Additionally, many of the techniques commonly applied to assess cryopreservation success are inferior based on modern day standards.
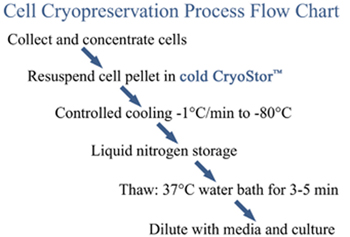
Cryopreservation
Cell cryopreservation can be a rather straightforward process, yet there are a number of protocol nuances that are critical for success. The primary examples include: 1) cryopreservation media, 2) temperature, 3) freezing profiles and 4) viability assessment. While all factors are important, recent studies have shown focus on the media is the most critical. Cryopreservation Media – While a mixture of serum/culture media/DMSO is common, this formulation is highly ineffective at preventing irreversible damage during the process. The use of a specially formulated cryopreservation media, such as CryoStor (BioLife), is recommended in order to provide for the greatest level of cell protection. Temperature – When adding the cryopreservation media to the sample it is important that the solutions be cold (∼4°C). Cell exposure to warm solutions containing DMSO can result in substantial cell damage and death. Freezing – Controlled cooling can be achieved most simply using a “Mr. Frosty” container (Nalgene-Nunc) and a -80°C freezer. For the best results, both the cooling apparatus and freezing solutions should be precooled to 4°C prior to utilization. Viability Assessment – Sample assessment (yield and viability) immediately post-thaw often yields significant overestimates in survival, whereas monitoring the population for at least 24 hours post-thaw provides a more accurate assessment of cryopreservation success.
Hypothermic or Cold Storage
Cells and tissues can be stored at refrigerated temperatures (∼4°C) for short periods (hours to days). These protocols can be optimized with the use of specialized media to prevent cold shock damage, such as HypoThermosol-FRS (BioLife). This third generation preservation media protects cells more effectively, extending viable transport/storage time on wet ice, ultimately providing enhanced time management of living samples.
Viability Assessment
Accurate sample assessment is critical to determining preservation success and downstream utility of cell and tissue systems, for both research and clinical use. TypanBlue assessment (or other membrane-integrity assays) immediately after thawing often yield elevated, inaccurate results due to the time dependent phenomena of preservation-induced delayed-onset cell death. Comparing samples to pre-freeze levels 1 day after thawing with metabolic or biochemical assays provide more accurate determinations of cell viability.